Welcome to Signalchem Lifesciences
SignalChem Lifesciences Corporation (SLC), a clinical-stage drug discovery and development company. Our extensive portfolio comprises cutting-edge, small molecule therapies specifically designed to target crucial proteins responsible for cancer initiation, progression, and treatment resistance with unparalleled precision. Leveraging our expertise in target biology, medicinal chemistry, and clinical development, we swiftly transform groundbreaking discoveries into potential treatments, instilling hope in cancer patients worldwide.
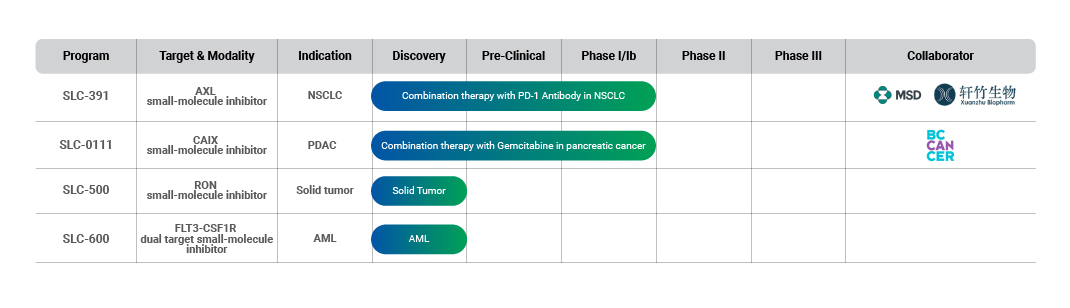
Pipeline
Investors & Partners
SignalChem is developing a solid portfolio of first-in-class and best-in-class treatments that target multiple mechanisms that underlie the non-responsiveness and resistance to targeted therapies. We work with strategic partners at every steps of the way - from the organizations who are interested in licensing our assets, to the contract research organizations and academics with whom we conduct our studies.
Learn More »